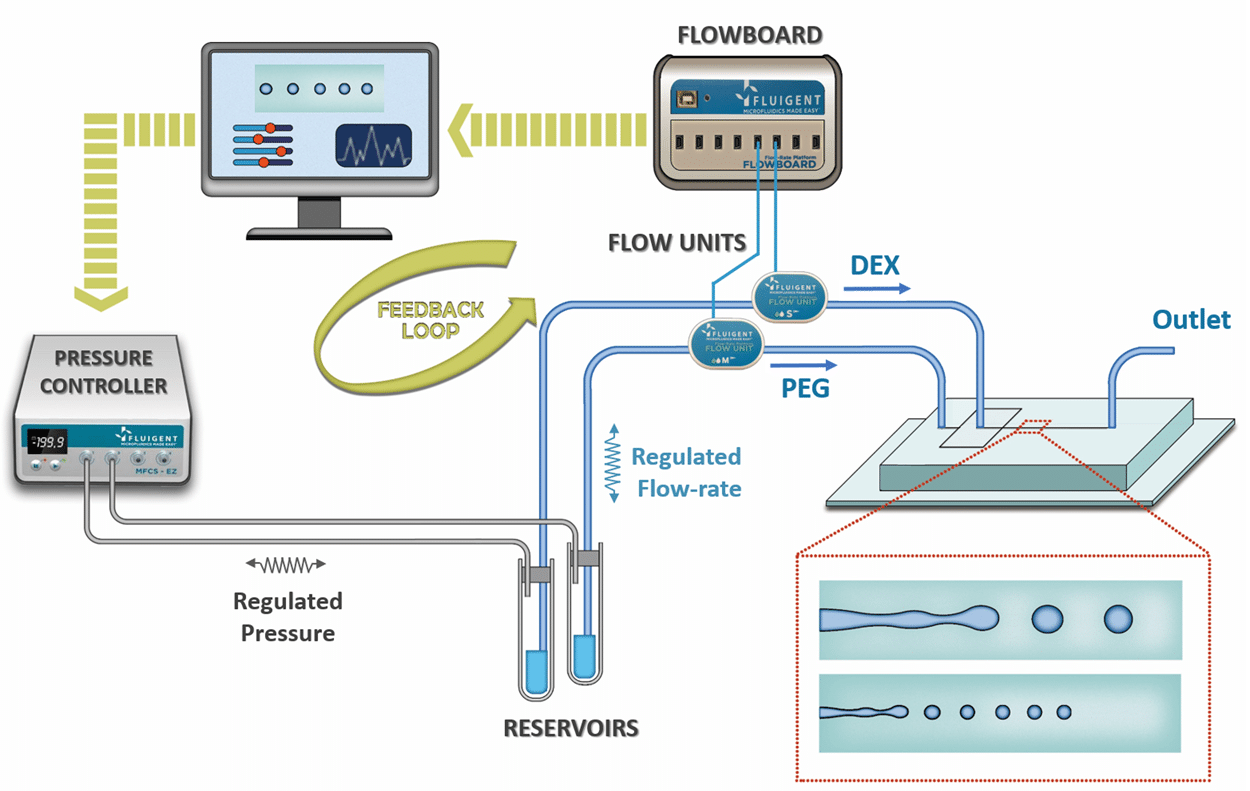
Microbead-based microfluidics (droplet-based microfluidic using specific gel as dispersed phase) is a powerful technic which…
Customer paper on particle tracking in blood flow
Toward Analysis of Proteins in Single Cells: A Quantitative Approach Employing Isobaric Tags with MALDI Mass Spectrometry Realized with a Microfluidic Platform
By Mian Yang, Randall Nelson and Alexandra Ros from the Arizona State University in the USA.
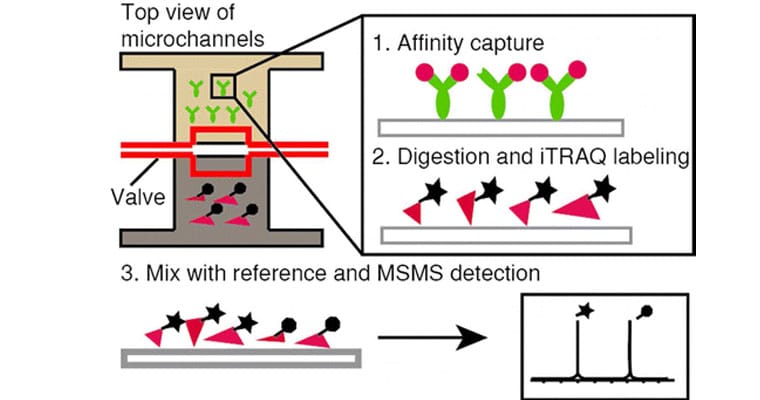
Protein identification and quantification in individual cells is essential to understand biological processes such as those involved in cell apoptosis, cancer, biomarker discovery, disease diagnostics, pathology, or therapy. Compared with present single cell genome analysis, probing the protein content of single cells has been hampered by the lack of a protein amplification technique. Here, we report the development of a quantitative mass spectrometric approach combined with microfluidic technology reaching the detection sensitivity of high abundant proteins in single cells. A microfluidic platform with a series of chambers and valves, ensuring a set of defined wells for absolute quantification of targeted proteins, was developed and combined with isotopic labeling strategies employing isobaric tags for relative and absolute quantitation (iTRAQ)-labels. To this aim, we adapted iTRAQ labeling to an on-chip protocol. Simultaneous protein digestion and labeling performed on the microfluidic platform rendered the labeling strategy compatible with all necessary manipulation steps on-chip, including the matrix delivery for MALDI-TOF analysis. We demonstrate this approach with the apoptosis related protein Bcl-2 and quantitatively assess the number of Bcl-2 molecules detected. We anticipate that this approach will eventually allow quantification of protein expression on the single cell level.
These experiments were made possible with the use our pressure-driven flow controller pumps to open and close microfluidic valves during the Bc1-2 quantification steps.
For more information: doi:10.1021/acs.analchem.5b03419 or Anal. Chem., 2016, 88 (13), pp 6672–6679.